Cell Types Promoting Goosebumps Form a Niche to Regulate Hair Follicle Stem Cells
Summary:
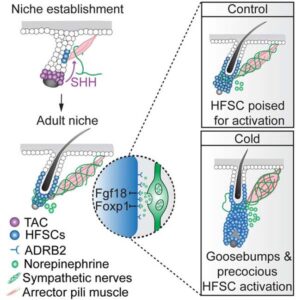
Piloerection (goosebumps) involves the coordinated actions of the hair follicle, the arrector pili muscle (APM), and the sympathetic nerve, offering a model to study interactions between the epithelium, mesenchyme, and nerves. In this study, we show that APMs and sympathetic nerves create a dual-component niche to regulate hair follicle stem cell (HFSC) activity. Sympathetic nerves form synapse-like structures with HFSCs, regulating them through norepinephrine, while APMs maintain sympathetic innervation to HFSCs. Without norepinephrine signaling, HFSCs enter a deep quiescent state, down-regulating cell cycle and metabolism, while up-regulating quiescence regulators like Foxp1 and Fgf18. During development, HFSC progeny secrete Sonic Hedgehog (SHH) to guide the formation of the APM-sympathetic nerve niche, which, in turn, controls hair follicle regeneration in adults. These findings reveal a reciprocal relationship between regenerative tissue and its niche across different stages and demonstrate how sympathetic nerves modulate stem cells through synapse-like connections and neurotransmitters, coupling tissue production with environmental demands.
eTOC/In Brief:
The arrector pili muscle (APM) and sympathetic neurons form a dual-component niche that regulates hair follicle stem cells. Sympathetic neurons regulate stem cells directly through synapse-like structures and norepinephrine, while APMs maintain sympathetic innervation to stem cells. Together, these three connected cell types ensure that cold stimuli not only trigger goosebumps but also activate stem cells.
Introduction:
Cells from multiple lineages come together to form specific arrangements in organs, and the integration of these cell types is essential for maintaining tissue homeostasis and function in a dynamic environment. The sympathetic nervous system (SNS), a branch of the autonomic nervous system, plays a crucial role in regulating body physiology during steady states and responding to external stressors such as cold or danger. Sympathetic neurons, which originate in the sympathetic ganglia near the spinal cord, extend axons that innervate virtually all organs, influencing processes like heart rate, respiration, and blood pressure.
In the skin, sympathetic nerves, the arrector pili muscles (APMs), and hair follicles form a tri-lineage unit. The sympathetic nerves innervate the APMs—bundles of smooth muscle cells attached to the hair follicle’s bulge region, where hair follicle stem cells (HFSCs) reside. Environmental stimuli, such as cold, activate this tri-lineage unit: sympathetic nerve impulses trigger the contraction of APM bundles, causing piloerection (goosebumps), a response that helps with thermoregulation by trapping air around the hair. While goosebumps have lost their thermoregulatory function in humans, the role of this tri-lineage unit in other physiological processes, such as hair growth, remains under investigation.
Recent observations indicate a link between sympathetic nervous system activity and hair growth. The hair follicle undergoes cyclical phases of rest (telogen) and growth (anagen). HFSCs in the bulge and hair germ are typically quiescent during most of the hair cycle but become proliferative at the onset of anagen, producing transit-amplifying progeny for hair growth. Loss of sympathetic innervation is associated with hair growth defects, and adrenergic agonists can promote anagen in cultured skin explants. Additionally, external light stimulation activates the sympathetic nervous system to promote hair growth.
These findings lead to several important questions: What are the direct cellular targets of the sympathetic nervous system in hair growth regulation? What molecular mechanisms enable sympathetic nerves to influence hair growth? And, given that APMs are part of the tri-lineage unit, what role do APMs play in regulating hair growth?
We address these questions using a combination of cell-type-specific gene deletion, cell ablation, transcriptome profiling, high-resolution imaging, and three-dimensional electron microscopy (3D-EM). Our results show that APMs are essential for the formation and maintenance of sympathetic innervation to HFSCs. This innervation activates HFSCs directly through synapse-like connections that release norepinephrine. Under basal conditions, this nerve-APM-HFSC connection primes HFSCs for activation by reducing the expression of quiescence regulators like Foxp1 and Fgf18. In response to cold temperatures, sympathetic nerve activity not only induces goosebumps but also accelerates HFSC activation to initiate hair growth, thereby coupling tissue regeneration with environmental changes. During development, Sonic Hedgehog (SHH) produced by developing hair follicles plays a role in the formation of this tri-lineage unit. Together, these findings provide insight into how cells from the epithelium, mesenchyme, and nerves work together to maintain tissue function and respond to environmental cues.
Results:
Sympathetic Nerve Activity Regulates HFSC Activity:
The sympathetic nervous system remains active at a basal level to support various physiological functions. To investigate whether basal sympathetic nerve activity affects the other two cell types in the nerve-APM-HFSC tri-lineage unit, we used the neurotoxin 6-hydroxydopamine (6-OHDA) to ablate sympathetic nerves in telogen skin. The 6-OHDA selectively ablated sympathetic nerves but spared sensory nerves and other skin cell types. Sympathectomy did not result in any significant changes in APMs but caused a notable delay in HFSC activation and entry into anagen. By postnatal day 30 (P30), hair follicles in control skin had fully entered anagen, while hair follicles in sympathectomized skin remained mostly in telogen. Similar results were observed in a genetic model of sympathectomy using TH-CreER; Rosa-lsl-DTA mice. These findings suggest that basal sympathetic nerve activity is required for HFSC activation and the initiation of anagen, though it is not necessary for the maintenance of APMs.